A More Comfortable and Stable Fit
Comfort and stability are important to helping you regain your activity level. The Mobile Bearing Hip™ Replacement is designed to help minimize the potential risks of hip dislocation and irritation of the muscles and tendons that support your hip.2, 3
Until now, in order to reduce the possibility of dislocation, doctors often turned to the use of metal-on-metal large head technologies for primary hip replacement. The specialized polyethylene insert of the Mobile Bearing Hip™ implant is designed to offer the potential benefits of a larger diameter head, which contributes to increased joint stability.2
Its specially designed cup is also less likely to impinge on the tendon that runs from the groin toward the front of the pelvic bone (iliopsoas tendon).3 An impingement might result in hip stiffness, groin pain and/or a clicking sensation in the hip — signs that the pelvis may be catching the tendon when the hip flexes. Stryker’s Mobile Bearing Hip™ features an anatomic cup with an iliopsoas tendon cut-out to help prevent impingement.

Less Wear and the Potential for a Longer Lasting Implant
Constantly looking to improve orthopaedic medicine, physicians and engineers study closely the “bearing surface” of implants — where the joint comes together to carry your body weight. Stryker’s Mobile Bearing Hip™ with X3® offers wear protection on two fronts. Both its dual mobility and its X3® technology bearing surface help to create a prosthesis that is designed to resist wear and has the potential to last longer.1 Test results demonstrate that X3® has shown a 97% decrease in wear compared to conventional polyethylene in laboratory testing.1 Less wear may mean a longer life for your hip replacement.
Wear Comparison1
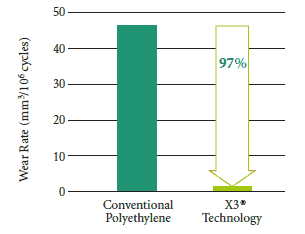
Talk to your doctor to see if Stryker’s Mobile Bearing Hip™ is right for you.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Mobile Bearing Hip, Stryker and X3. All other trademarks are trademarks of their respective owners or holders.
References:
1. Stryker® Orthopaedics Trident® Acetabular Inserts made of X3® UHMWPE (unsterilized), 721-00-32E, show a 97% reduction in volumetric wear rate versus the same insert fabricated from N2\Vac™ gamma sterilized UHMWPE, 620-00-32E. The insert tested was 7.5mm thick with an inner diameter of 32mm. Testing was conducted under multi-axial hip joint simulation for 5 million cycles using a 32mm CoCr articulating counterface and calf serum lubricant. X3® UHMWPE Trident® Acetabular Inserts showed a net weight gain due to fluid absorption phenomena but yielded a positive slope and wear rate in linear regression analysis. Volumetric wear rates were 46.39 ± 11.42mm3/106 cycles for N2\Vac™ gamma sterilized UHMWPE inserts and 1.35 ± 0.68mm3/106 cycles for X3® UHMWPE (unsterilized) Trident® Acetabular Inserts. Although in-vitro hip wear simulation methods have not been shown to quantitatively predict clinical wear performance, the current model has been able to reproduce correct wear resistance rankings for some materials with documented clinical results.a, b, c
a. Wang, A., et al., Tribology International, Vol. 31, No. 1-3:17-33, 1998.
b. Essner, A., et al., 44th Annual Meeting, ORS, New Orleans,Mar. 16-19, 1998:774.
c. Essner, A., et al., 47th Annual Meeting, ORS, San Francisco, Feb. 25-28, 2001:1007.
2. Stryker Test Report: RD-09-068.
3. Tracol P, Vandenbussche E, Deloge N, et al., Navigation Acetabular Anatomic Study Application in the Development of a New Implant. EFORT Poster, 2007.
