System Description
The Trident® Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The artificial hip replacement device consists of four basic components: an alumina ceramic insert (socket liner), an alumina ceramic femoral head (ball), a metal acetabular shell (socket), and a metal femoral stem (hip stem).
The hip stem is inserted into the top of the thighbone. The ball fits onto the top of the hip stem. The socket liner and mating socket are fixed to the hip joint. The ball and socket articulate together.
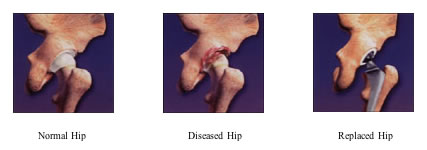
The Trident® implant has bearing surfaces (the ball and socket) made of alumina ceramic. Laboratory testing of alumina ceramic has shown it to have less wear than the metal and plastic materials that are currently used in total hip surgery. Alumina ceramic is extremely hard — in fact, its hardness is second only to diamond — and provides excellent lubrication between the ball and socket. Because of its material characteristics, alumina ceramic-on-ceramic demonstrates significantly lower wear versus conventional metal-on-plastic components in laboratory testing. Therefore, it is anticipated that these improved wear characteristics will extend the life of the implants.
Purpose of the Device
This total hip replacement is indicated for patients with painful, disabling hip joint disease caused by a severe form of arthritis.
This total hip replacement should not be used for patients:
- Who may have an infection in or near the hip joint,
- Who are unable to comply with the instructions for preparing for and recovering from the surgery,
- Who do not have enough healthy bone to support fixation of the implants, or
- Whose bones are not fully grown.
Clinical Experience
Howmedica Osteonics Corp. (hereafter referred to as Stryker Orthopaedics) conducted a clinical study in the United States for its ceramic-on-ceramic hip system, which was conducted under a study protocol approved by the United States Food and Drug Administration. The study was performed at 16 orthopaedic centers of excellence across the United States. The clinical study was conducted for Stryker Orthopaedic’s first generation ceramic-on-ceramic design (called the ABC System), and second generation ceramic-on-ceramic design (called the Trident® System). In all, the data from 558 hip replacement surgeries (cases) with these ceramic-on-ceramic bearings were evaluated.
The prospective, randomized, clinical study began in 1996, with 349 cases with the first-generation ceramic-on-ceramic systems, and 165 cases with a control device (conventional, metal-on-polyethylene). The clinical data on these cases were evaluated out to 24 months (2 years) after surgery. In 1999, the patented Trident® Ceramic System was then entered into the study. This patented design offers a stronger, easier-to-use ceramic liner design. Two hundred nine (209) Trident® inserts were implanted in the study. The clinical data from these cases has also been evaluated out to 24 months (2 years) after surgery.
The Trident® System features the identical ceramic-on-ceramic articulating bearing surface as the ABC System; however, the Trident® system features an outer titanium sleeve. The titanium sleeve offers the following unique advantages over other first generation ceramic-on-ceramic designs:
- Increases the strength of the ceramic insert by 50%: The Trident® Alumina Insert is the strongest ceramic insert on the U.S. market today,
- Protects the insert rim from chipping during implant assembly: Occasional chipping of the insert rim during surgery was reported during clinical studies of first-generation designs. No Trident® ceramic implants chipped or fractured in the study.
- Allows reassembly of the acetabular shell to the insert: First-generation designs prohibit reassembly of a ceramic insert to its shell, making insert adjustment or replacement more difficult.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Stryker and Trident. All other trademarks are trademarks of their respective owners or holders.
Next Page >>